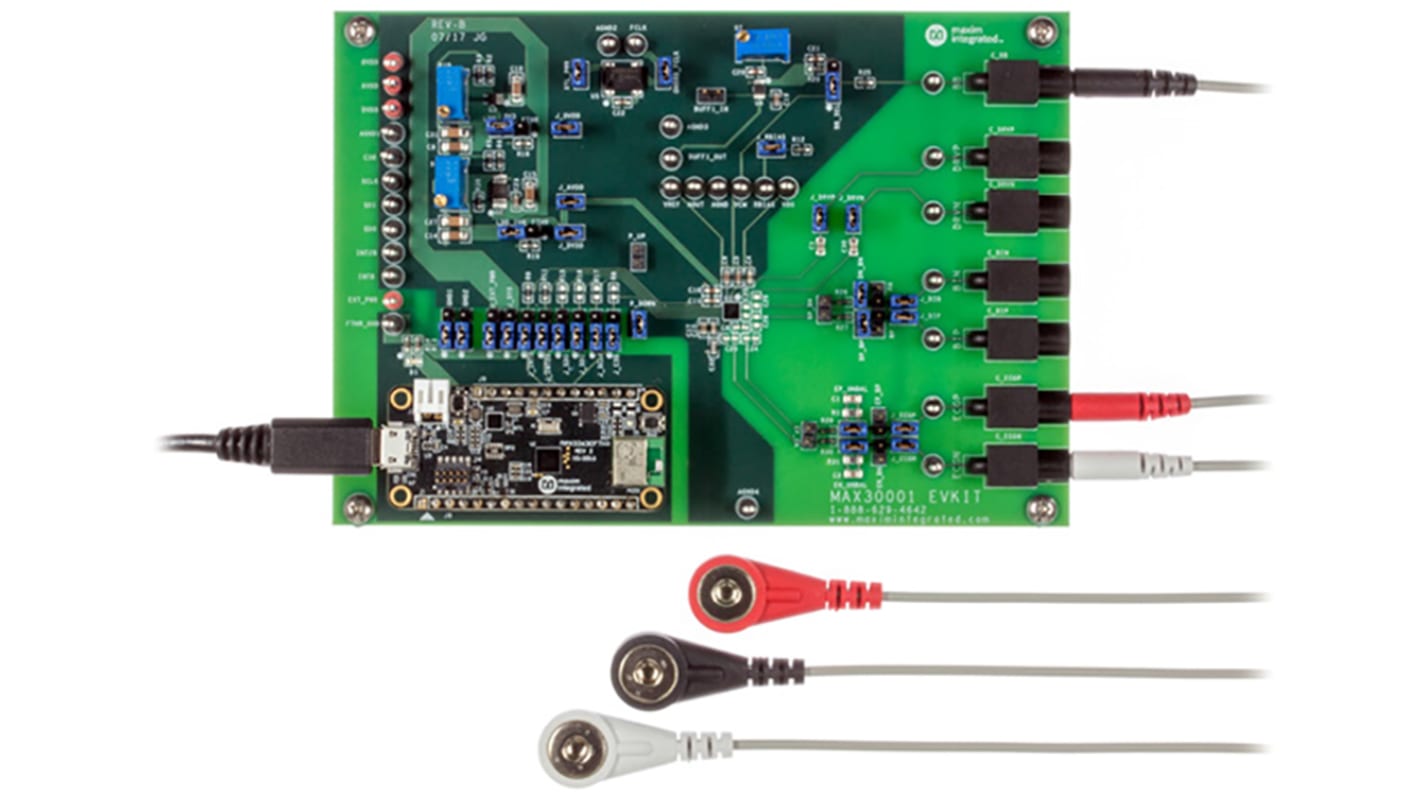
Maxim Integrated MAX30001EVSYS# MAX30001 Evaluation System Evaluation Kit Evaluation kit
- RS Stock No.:
- 191-4218
- Mfr. Part No.:
- MAX30001EVSYS#
- Brand:
- Maxim Integrated
Stock information currently inaccessible
- RS Stock No.:
- 191-4218
- Mfr. Part No.:
- MAX30001EVSYS#
- Brand:
- Maxim Integrated
Specifications
Technical Reference
Legislation and Compliance
Product Details
Find similar products by selecting one or more attributes.
Select all | Attribute | Value |
|---|---|---|
| Brand | Maxim Integrated | |
| Kit Classification | Evaluation Kit | |
| Featured Device | MAX30001 | |
| Kit Name | MAX30001 Evaluation System | |
| Select all | ||
|---|---|---|
Brand Maxim Integrated | ||
Kit Classification Evaluation Kit | ||
Featured Device MAX30001 | ||
Kit Name MAX30001 Evaluation System | ||
Convenient Platform to Evaluate the MAX30001
Many Easy-to-Reach Test Points
Measure Individual Supply Currents
Touchproof Cable Connectors
Windows® 7/8/10 Compatible GUI software
Fully Assembled and Tested
Facilitates IEC 60601-2-47 Compliance Testing
Ultra-Low-Power Design
Applications
Bio Authentication and ECG-On-Demand Applications
Chest Band Heart Rate Monitors for Fitness Applications
Impedance Based Heart Rate Detection
Respiration and Hydration Monitors
Single-Lead Event Monitors for Arrhythmia Detection
Single-Lead Wireless Patches for In-Patient/Out-Patient Monitoring
Many Easy-to-Reach Test Points
Measure Individual Supply Currents
Touchproof Cable Connectors
Windows® 7/8/10 Compatible GUI software
Fully Assembled and Tested
Facilitates IEC 60601-2-47 Compliance Testing
Ultra-Low-Power Design
Applications
Bio Authentication and ECG-On-Demand Applications
Chest Band Heart Rate Monitors for Fitness Applications
Impedance Based Heart Rate Detection
Respiration and Hydration Monitors
Single-Lead Event Monitors for Arrhythmia Detection
Single-Lead Wireless Patches for In-Patient/Out-Patient Monitoring